QA/QC & PI
AUTOMATION
Precision Verification & Workflow Acceleration
The Challenge
Manual data transfers coupled with manual QC/QA are significant risk factors that impact quality and compliance. QC logging and data entry for large studies are notoriously time-consuming, often leading to fragmented information and delayed report releases.
Fragmented data across sub-systems like Watson, Quickbase, and SharePoint e-Binders creates "information chaos," making it difficult to consolidate numbers and identify correct data points when mismatches occur.
OVER 99% ACCURACY
Automated outlier detection and data validation achieving over 99% accuracy to streamline human review.
UNIFIED DATA
Seamlessly pull and compare data from Watson, Quickbase, and SharePoint to resolve mismatches instantly.
GLP COMPLIANT
Ensure compliance with GLP standards through automated checklists and signature verification.
Key Capabilities
-
▶
Outlier & Deviation Detection
Instantly identify data points that fall outside expected ranges or violate study protocols.
-
▶
E-Signature Verification
Automated detection of missing signatures from chemists and PIs to prevent submission delays.
-
▶
Cross-System Validation
Compare sample counts and primary data across Watson, Quickbase, and IDBS e-Workbooks.
-
▶
PI Pre-Review Tool
Empower Principal Investigators to identify issues before formal QA submission, reducing cycle times.
Business Benefits & ROI
Accelerated Report Release
Reduce the time from study completion to report submission by automating repetitive verification tasks.
Reduced Operational Risk
Eliminate manual data entry errors and ensure all regulatory requirements are met before QA review.
Resource Optimization
Free up highly skilled QA/QC professionals to focus on complex judgment-based reviews rather than tedious data checks.
SYSTEM INSIGHTS
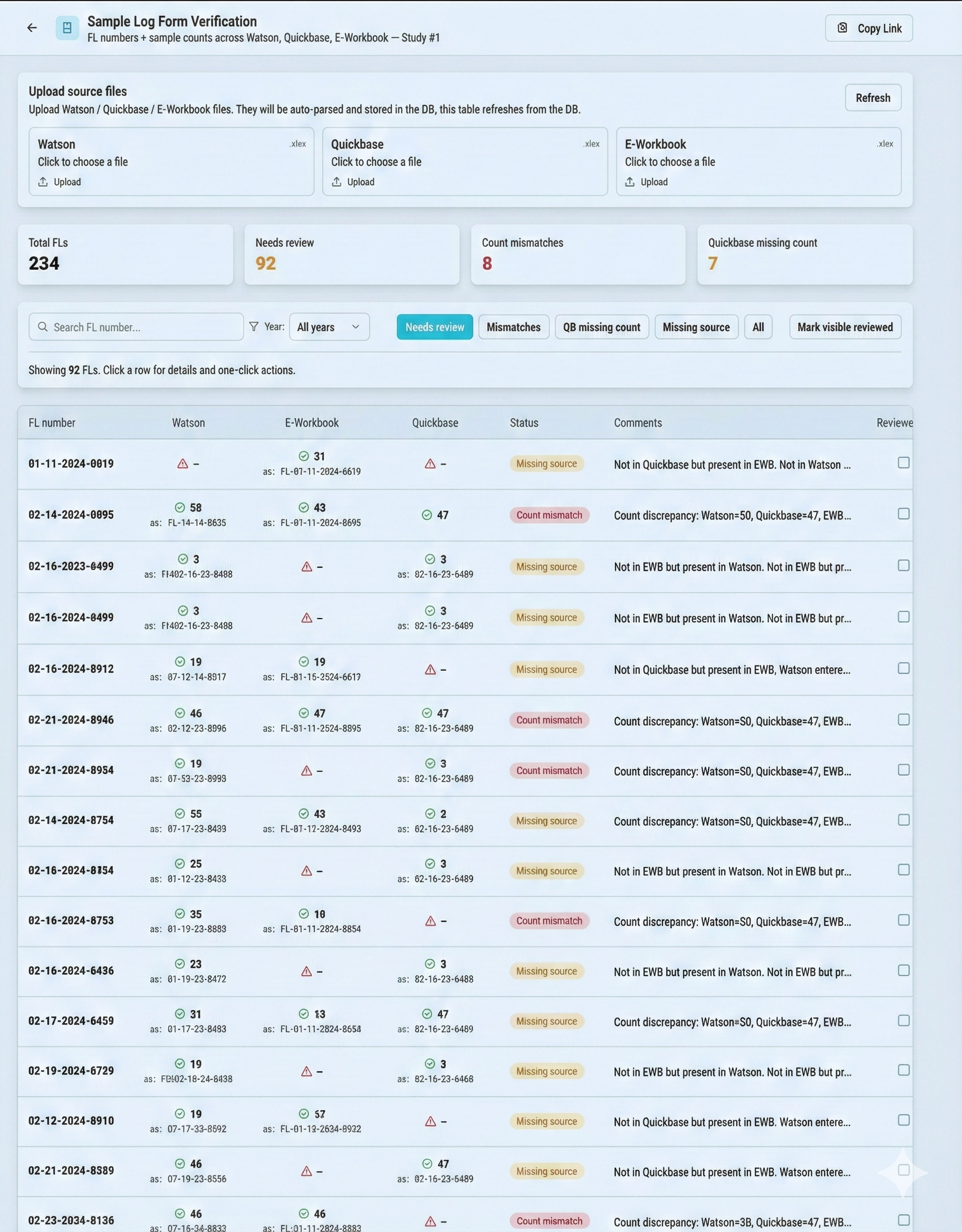
VERIFICATION DASHBOARD

Data Mapping & Ontology

Validation Checklist
SYSTEM INTEGRATIONS
Automated data ingestion, extraction, and semantic verification.
Real-time synchronization for automated sample tracking and Quickbase intake forms.
Direct e-Binder access for comprehensive document review.
e-Workbook integration for seamless experiment data validation.